Abstract
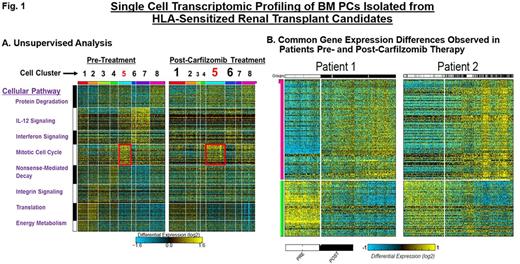
Kidney transplantation improves the overall survival of end stage renal disease patients but the presence of preformed human leukocyte antigen (HLA)-specific antibodies often precludes finding an acceptable donor. Recent studies demonstrate that bone marrow plasma cells (BMPCs) are a major source of HLA-specific antibodies in highly sensitized transplant candidates. However, therapeutic strategies that target BMPCs and overcome HLA desensitization are only beginning to emerge. Proteasome inhibitor (PI) therapies effectively eliminate BMPCs, reduce HLA-antibody levels and have shown promise as an effective means of enhancing transplantation outcomes. However, PIs are frequently limited by de novo or therapy-induced drug resistance. In addition, BMPCs exhibit variable sensitivity to PI-based therapy. To identify potential mechanisms of drug resistance in BMPCs, we employed single-cell genomic profiling. Single-cell studies provide a powerful tool that allows us to study rare cell populations, such as BMPCs, and intermediate cell states that cannot be resolved at the population level. Here, we have performed single-cell genomic profiling of human BMPCs isolated from highly HLA-sensitized renal transplant candidates prior to and after desensitization therapy with the irreversible PI carfilzomib. HLA-sensitized transplant candidates received CFZ (20, 27, 36 mg/m2) with BM aspirates performed prior to and after CFZ therapy. CD138+BMPCs were isolated to >98% purity and individual cells analyzed using the recently developed method, Iterative Clustering and Guide-gene Selection (ICGS), which utilizes pair-wise correlation of dynamically expressed genes and iterative clustering with pattern-specific guide genes to delineate coherent gene-expression patterns. Strongly and differentially expressed gene sets were clustered and evaluated using gene enrichment, prior knowledge-based networks and ICGS. Each cluster corresponds to one or a group of closely related cell types that represent spatial or temporal distinct states. Single-cell profiling by the drop-seq method analyzed mRNA expression in 2,000 individual BMPCs isolated from the same HLA-sensitized patient prior to and after CFZ therapy. We used CellHarmony, a new bioinformatic approach that allows us to determine the frequency of all CFZ-treated cells in direct reference to the untreated cells and any altered gene expression profiles. In our first comparison of these cells, eight cell clusters were defined (Fig. 1A). Single-cell analysis of BMPCs obtained post-CFZ therapy indicated qualitative and quantitative changes in gene expression as well differences in the proportionality of existing cell clusters. To determine the frequency and expression of these genes in the CFZ-treated samples, we then performed classification of those samples using a k-nearest neighbor approach using all untreated cells as the reference. For example, protein catabolism, nonsense-mediated decay and integrin signaling were increased (clusters 1, 5 and 6) while clusters enriched in IL-12 signaling, interferon signaling and protein translation genes (2, 3 and 7) were reduced. Importantly, the state associated with the mitotic cell cycle was significantly upregulated in BMPCs that survived CFZ. BMPCs were analyzed from additional patients using a comparable approach identified common gene expression sets in pre-treatment samples as well as recurrent differences in post-treatment samples (Fig. 1B).
Conclusions: The results presented here demonstrate the power and promise of single-cell genomics in uncovering functional diversity between cells and in deciphering how cell states respond to therapeutic challenge. Our results indicate a shift toward highly mitotic states in BMPCs that survive CFZ therapy. Importantly, the single cell results also provide internal validation of our prior bulk RNA-seq studies on BMPCs. Our results suggest the feasibility of pathway-based secondary targeting of the recovering cell populations to enhance response.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal